My cancer has taken an ugly turn. I will update more if able. Been in hospital since Oct 20th. Hoping things are turning back around.
The Cancer Classroom
Wednesday, November 17, 2021
Friday, April 2, 2021
Featured on Everyday Health Website
At the end of January, a video production company interviewed me and my daughters for a possible feature on a website called Everyday Health. It was quite a surprise when I was contacted a few days later to inform me I had been chosen. My daughter, Audrey, spoke for the family.
The first video is called What my Mom's Metastatic Breast Cancer Means to Me; the second is called Diagnosed with Breast Cancer the Third Time Around.
The videos produced were released on March 18th. There are two. Click the links below to see the videos. You will be redirected to the website.
While you're there, explore the website.
Thanks for watching!Sunday, March 28, 2021
Implants No More
A few weeks ago, after showering, I noticed a dark area underneath my artificial breast on the right side. I wasn't actively looking for anything, I just happened to notice it when I glanced in the mirror. Of course, I immediately touched it. The darkened area was soft where the implant was pushing outward. It didn't hurt unless I pressed really hard. The rest of the implant felt hard. The skin on that side had a darker hue than the left.
"This has to be bruising," I thought, "but I haven't bumped into anything recently to cause it."
To the internet I went. This had to be Capsular Contracture.
Here is the definition of Capsular Contracture from Wikipedia: The occurrence of capsular contraction follows the formation of capsules of tightly-woven collagen fibers, created by the immune response to the presence of foreign objects surgically installed to the human body, eg. breast implants, artificial pacemakers, orthopedic prostheses. . . . Capsular contracture occurs when the collagen-fiber capsule shrinks, tightens and compresses the breast implant. (It is the immune system trying to protect the body.)
The right side of my chest had been tighter than the left for years. However, recently, I had noticed some minor pain that would come and go. Plus, now the right side implant sat noticeably higher than my left.
After some thought, I decided to call the plastic surgeon's office. It was a Sunday meaning I would have to speak to the surgeon on-call, which was fine. All I I needed was someone to tell me this was not an emergency situation.
My implants were placed in 2005. Since then I have had radiation over the whole area and two other more targeted radiation treatments in that same area. Radiation is probably the reason for the implant's demise.
The doctor on call that day made me feel at ease; there was no hurry to get them removed. He asked that I call the office to make an appointment.
The appointment was made.
As I put on the fashionable paper vest and waited, I thought about how I wished I wasn't there. Yeah, the mask was hiding my looks, somewhat, but I couldn't forget what my plastic surgeon said in 2005 when I was early stage.
He said, "I am so glad you don't have to do Chemotherapy. So many people look so old afterwards."
Isn't that a nice memory to hold on to?
My plastic surgeon arrived, took one look--didn't even touch the bruised area--and said, "Yeah, that needs to be removed."
Good grief, it seems like every time I think I am through with medical procedures on my scared-up body I have to undergo another one.
"We could do this or that," never entered the conversation. I had one choice for my capsular contracture: take it out.
I did have a choice about the left one: take out it out or leave it. I am a fan of symmetry, so I chose the latter.
The procedure was done on March 12th. Now my 90 pound frame, looks like a prepubescent female. Thanks, cancer.
When I got home, I again revisited 2005 when I underwent bilateral mastectomies. I had forgotten how unpleasant it was to have drains inserted under the skin. Any fluid made by my body as it healed traveled down a plastic tube and into a collection bulb, gross. I wasn't in a lot of pain, thankfully--only took one prescribed oxycodone pill, but boy did my skin itch. I was miserable! Since the procedure was done Friday afternoon, I had a few days to figure out what I was going to wear for work to hide my shrunken chest and to figure out how to hide those awful bulbs at the end of the drains. Monday came, and I decided the best thing for me was to remove the collection bulb at the bottom and tuck the tubes into my pants. I used the cap on the bulb to close off the tubes.Since there wasn't a lot of drainage, the two drains were removed on Friday. After the mastectomies, I had 4 drains. Those stayed in much longer.
I know the image below is ugly, and I might regret showing it--but this is the Cancer Classroom. I wanted to show what the tubing and the bulbs looked like. Images can be more impactful than words at times. I think that is true here.
Cancer treatments damage a person's body in a variety of ways. The bluish area at the center top is radiation damage. There is a lot more of that above it. The scars above and around my belly button area are three out of the four I have from the hysterectomy in November--for me, a cancer preventative surgery.
As the following week moved along and the next week too landing me here, Sunday March 28th, I noticed and could feel my lymph system at work. There was fluid accumulating; my body couldn't keep up. The right one, in particular, felt like a balloon filling with water. Maybe I should have left the drains in longer?
On Friday, I called the nurse. An appointment was made. There, the fluid will be extracted. In the meantime, I am wearing a tight athletic bra. I have placed soft padding over the fluid filled areas to create pressure to hopefully slow down further fluid accumulation and the to help my body absorb some of the fluid already present.
Just another day in the life of a metastatic breast cancer patient.
Sunday, February 21, 2021
Scan Update
Well, it is over. I made it through another scan.
To recap: Three months ago I learned that a lymph node toward the front of my chest had enlarged to 1.9 cm--that is huge! Those guys are usually not even a centimeter in size Also, there is some thickening of the pleura of my right lung that the radiologist deemed potentially metastatic spread.
As I usually do, my brain went through the different scenarios preparing for what could be the worst or even the best results of this scan. I would think about what my oncologist would say. Would she begin with: how are you feeling--an indication the news was not good. Or, would she jump right in with the only information that mattered--an excellent start for what will most certainly be great news. Then I would play my mind's movie, what I would say and how I would say it. In the past, I have had some kind of plan. A plan I could throw at her hoping she catches it in order for me to stay on my current treatment. Luckily, in the past, she has caught it, agreed to it, and the plan worked. But, my research and the knowledge that I have already had so much radiation to my chest made me painfully aware this time I had no plan. My sense of control--so needed by all of us--was gone.
The knock came, my oncologist entered. She seemed at ease, a good sign. She sat down and began talking. "Well, your scans look good".
I say, "What?"
She looked at me like: what do you mean, what?
That followed with the best conversation. A conversation I was so sure I was not going to have.
She let me know that the lymph node had decreased. Could have been some kind of infection causing the increase but really all that mattered was its size. It wasn't super small, yet, but it was down from 1.9 to 1.5 cm. As for the pleural thickening, it was stable. She didn't seem that concerned about the thickening having not mentioned it to me two months ago. I learned of it by reading the radiologist's comments on my report.
We moved away from the scan report as our conversation continued. I asked her whether I should get the COVID vaccine. She said, "Yes", without hesitation.
My next scan was set and will be at the end of May just before school lets out for the summer. And, I get to continue my treatments with Kadcyla. The burden was lifted. and I smiled all the way to the infusion room.
Thinking of spring and all of life's possibilities as I continue to find my way in the world after leaving my husband made for a tremendously happy ride home. If Kadcyla can continue doing the incredible task of keeping those little cancer cells in check, maybe I will be watching my youngest graduate from college in 2025. Hope is still winning!
Thursday, December 31, 2020
If it's not one thing . . .
I am sitting here staring at my computer hoping the words will come easily. It has been awhile since I have written on this blog, or anywhere, really. COVID isn't the only thing in my life that has made 2020 an extremely crap ridden year. I sit. I stare. I wait. I know that if I would just let go and allow words to spill onto this page, I could then shape it into what I want to say. It is hard but here goes.
After genetic testing revealed that I have Lynch Syndrome, see this post, and because of it I am at a greater risk for certain cancers compared to the general population, I had to make a decision: keep my uterus, cervix, ovaries/ tubes, or have surgery to remove them. I chose the latter.
I also was advised to have a colonoscopy. So, I did that too, in October. I had been avoiding having a colonoscopy because my oncologist and I talked about it years ago. At that time the thought was breast cancer would kill me before colon cancer would and surveillance was occurring anyway every three months by the CAT scans. I finally caved when a gastroenterologist from UNC-Chapel Hill thought it would be a good idea. One precancerous polyp was found, so I do feel better about having it done. The two-day prep was awful, and if I am still around in 2 years I am supposed to have another.
The newest doctor(s) in my life, a GYN oncologist and her team, recommended I have a complete hysterectomy and an oopherectomy. I waffled back 'n' forth, trying to figure out what was best for me. The possibility of being diagnosed with another type of cancer or my breast cancer metastisizing to my uterus or ovaries helped me decide. I want to live as long as I can, so under the skilled hands of a surgeon doing robotic surgery, I sit here now with no uterus, ovaries, or fallopian tubes. My belly has finally returned to its normal size.
Two days before the big event, I was asked to go through pre-op which involved collecting my blood to look at white blood cell and platelet counts. A nurse collected blood from the inside of my elbow. I have never had a nurse who was so forceful at collecting those vials of blood. I mean she shoved the needle in and pressed hard in order to get the blood to flow quickly. When she was finished, she placed a piece of gauze where the needle had penetrated my skin, added tape to hold it in place and sent me on my way. I had another appointment during which I would learn about the procedure. When I reached a desk for check-in, I felt a warm, wet sensation coming from my left arm which was inside my coat. Separating myself from my coat I discovered blood spilling out from under the gauze. Since this was not a little bit of blood, I panicked just a wee bit. I asked for help and the young woman sitting at the desk ran to find someone to help me. She seemed to be taking way too long in her quest that all I could do was apply pressure to the area with the small blood saturated gauze taped to my arm and cry. Yep, stood there and cried. The help I needed arrived, and after leading me to a back room, the bleeding was stopped.
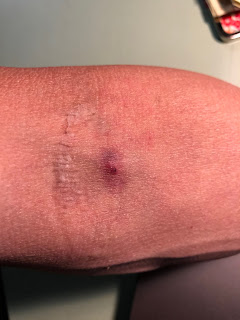 |
| my arm the day after the blood draw |
November 25th arrived--the day before Thanksgiving. The day of my oophorectomy and complete hysterectomy.
Afterwards, a doctor came by my room. I was told about the complication which explained the need for the catheter to remain in my bladder for a week. While the surgeon was telling the robot where to cut and when to cauterize a blood vessel, I learned that a small puncture wound occurred damaging my bladder. She explained that I had a lot of scar tissue from the two c-sections that brought two of my children into the world. There was so much scar tissue she said that my bladder and uterus were "cemented" together. The hole was repaired, and I was so grateful to be alive--one of my greatest fears is not waking up after a surgery. I stayed overnight in the hospital and was released the next day. As you can imagine, walking around in public with a foley bag attached to your leg does not equate to feeling good about yourself. And yes, I wore long pants.
I was discharged from the hospital on Thanksgiving day. All went well over the next week--so thankful I didn't have to work. Soon I was back at UNC for a voiding cystogram where a contrast was injected into my bladder. The technician asked me to hold as much of the contrast inside my bladder as I could in order to make sure there was no leakage. My bladder inflated as it was supposed to and no leaks were found, so the catheter was removed. Ah, freedom!
The next Saturday, I had an infusion. It had been six weeks since my last one--delayed in the hopes my platelet count would increase prior to surgery which it did.
By Sunday, I had burning and pain with urination that actually had started as soon as the catheter was removed, but now it was affecting my quality of life. The pain was tremendous and getting up through the night and feeling like I had to urinate every hour caused me to go to a clinic on Sunday. There I was given antibiotics for a UTI (urinary tract infection). I should have found relief a few days later, but no, I was still in pain.
My UTI wasn't getting any better causing me to make an appointment to see one of the doctors on my surgeon's team. Before addressing my UTI, she examined me and found an area in my vagina that was irritated and not healing as it should. (I wonder if the the tools used to remove my uterus via the vagina may have caused this injury. When you don't have a lot of estrogen in your body, the vagina does become dry and less pliable--purely my speculation since no one has offerred any explanation, but it makes sense to me.) Also, the doctor found a small blood clot of which she removed. Then she placed some silver nitrate on the wound to try to control the bleeding. (It was not heavy bleeding, thankfully.) The antibiotic I was taking was changed. Within a few days, I thought I was getting better, although ever so slowly.
But alas, my UTI proved to be complicated. I received a phone call few days later from one of the doctors on the team. She said my urine culture was back. It showed that the particular bacteria causing my UTI was oral antibiotic-resistant. She asked that I come to the hospital and be admitted for IV antibiotics that night. So, I took care of a few things, and away I went back to the hospital.
Oh, the miles my newish car was traveling. (I bought it October 19, 2020.)
I spent one night in the hospital where I received two doses of the IV antibiotic. The rest of the medication was to be given by me through my port at home. Once I was cleared to leave, I hurried home. The drugs were to be delivered to my home at 8pm that night. I waited and waited. Eight o'clock became eight thirty. I tried calling the company responsible for the delivery but despite the internet telling me this business was open, no one would answer the phone. I then decided I would go to my 24-hour pharmacy, CVS, and pick up another prescription drug I needed. Well, for some reason unknown to me now, I decided to call the pharmacy. That is when I learned CVS's pharmacy is not 24-hour right now (thanks COVID) and would not be open after 9pm and due to my waiting for my drug delivery, I missed the chance to arrive at CVS in time. So I went to bed, mad.
I called the company responsible for the drug delivery the next morning. For whatever reason, no one would answer my call at 8:30 a.m. They, again, were supposed to be open. Out of frustration I called my GYN's office and asked for help. A home health nurse was supposed to be coming to show me all the steps for administering the drug. But, what good would that do me if the items needed had not arrived?
An hour later, I heard back from my doctor's assistant. She was getting things moving since some miscommunication (or actually no communication) between people had occurred. The drug was now scheduled for delivery by 1 p.m.
In the end, the problem was resolved. The drugs were delivered and a home-health nurse came to see me. (She was notified at the end of her shift that she had one more patient to see, me.) I am grateful she went out of her way to help me. When she arrived, she apologized for the what should have never happened with my care.
She then proceeded to explain what I should do concerning administering the antibiotic, the saline, and the heparin through my port. I admit it was unnerving. I didn't want to do it wrong and something bad happen to me because of my own stupid mistake. Once I got the hang of it, it wasn't hard to do. By Saturday, I gave myself the last dose. A different home-health nurse came by late that afternoon and de-accessed my port. It was nice having that tubing that dangled from my port gone. Now, I could take a long, hot shower without worrying about getting my port wet.
 prep--work done before injecting drugs: saline, antibiotic, saline, and heparin plus all necessary alcohol wipes used at the end of the tubing. | ||
During this time I began wondering what is going to go wrong next. It is 2020 after all.
I had a CAT scan on December 7th to check for any progression of cancer in my body. My oncologist called me the same day but not with any results concerning cancer. She wanted me to begin taking Eliquis for three months because a small blood clot was found in my left lung caused by deep vein thrombosis (DVT). Not an unusual complication after abdominal surgery. Precautions against this happening were taken. I sported lower leg messagers while in bed. Plus a blood thinner had been given. Yet none of that helped. Eliquis is now added to my growing list of drugs to be taken daily. In three months I will no longer need to take it, so I am told. It is supposed to prevent any future clots and to help my body break-up the one in my lung. Since I was still bleeding from my vagina, it was decided I wait a few days before I begin taking it.(Another thing to worry about. Whew! I need a break.) During our conversation, I asked if she could call me about my scans even though I was scheduled to see her on Monday. I expressed that scans done on the 7th and then discussion of them not happening until the 14th is way too long to wait. I have never had to wait that long, ever! For years now I have done the scans then saw her to discuss the preliminary scan report and if all was great, I would head to infusion. All of this was done in one day and now she wanted to do it over two days. She was quite resistant about calling me. Instead she wanted to see me in person on the 14th. Of course this made my brain jump to all sorts of conclusions. (Surely she already knew what was going on in my body. I mean she spoke to the radiologist over the phone. Why would they not speak of any cancer spread?) Somehow, in the end, she agreed to call me when she had the completed report. As it turned out, the telephone appointment would have had to occur anyway. I was in the hospital for the UTI on the 14th--the day my appointment with her was scheduled.
News of my scan report came a few days after my telephone call about the blood clot. The words enlarged lymph node in the front of my chest along with some plueral thickening of my right lung suspicious for metastatic spread just takes the joy of believing there is a future I can continue planning. All my dreams began to vanish. And, now I must wait for another scan to confirm progression or not. That will be done on February 5th. I will meet with my oncologist a few days later for the results. The year 2020 has truly sucked for me and not just because of COVID. Just one bad thing after another.
- February-- Radiation to medistinal nodes
- January--I was told I had Lynch Syndrom which is a genetic condition which makes a person more susceptible than the general population to certain types of cancers.
- March--I left my husband.
- August--Human Resources and my principal were trying to figure out what to do with me during my school's shut-down. I was offered half my disability for one year (no one could live on that). I am only eligible for half since I haven't worked there long enough for full benefits. At one point I felt like I was being pushed out of my job after my doctor said I needed accommodations made and that working from home was her recommendation. I submitted what accommodations would be needed showing everything I could do from home, but those were rejected by HR. During the conversation that seemed like they were telling me I would be let go if I could not work on campus. I mentioned--through tears--I had a BA in elementary education. With that the woman I was speaking with said she would call back. Well, instead of her calling it was the Chief Officer. Their tune had changed and accommodations were made. So, for the first part of the new school year I worked at home calling parents, providing technology assistance, and working with students on-line until mid October.
- Hysterectomy and Oorechtomy
- Possible progression
With all the trouble my hysterectomy has caused me, I was told no cancer was found in the uterus or ovaries. That is great! except for the one part of me thinking, "Wow, if I had known that I wouldn't have gone through with the surgery." But, hindsight is 2020 in this crazy year of 2020.
The day after Christmas, I had another infusion. I truly need Kadcyla to get to work and not allow any cancer cells to be resistant to it. The word "worried" isn't adequate to explain how I am feeling about the possibility of moving on to the next in-line drugs. At this point, radiation to the enlarged chest lymph node or the thickening of the pleura in my right lung isn't a possibility nor is removal of these tissues. In the past, when progression occurred, I had radiation as an option to stop the cancer cells. This time I feel like I am falling into the abyss with no way out. All my future plans are a fantasy even though my oncologist said she wasn't convinced that progression was occurring. I am trying to side with hope. But lately, fear has been in charge.
I will end this post with the three things that have truly made this year bearable: My youngest daughter, my pupster, Dashiell (aka Dash), and my newish car that replaced my 17 year old van with 400,109 miles driven.
Sunday, August 2, 2020
Lynch Syndrome
 |
| digitphotos.com |
Sunday, June 7, 2020
A Guessing Game
All those painful, scary, heavy, and unwelcome emotions got up and walked away on May 25th after receiving the phone call from my nurse practitioner. Phew!
She said, "There is good news! The spot on your pelvis did not show up on the bone scan."
What a sense of relief I felt!
Then she says, "But . . . ."
Gotta say I have had enough with the word BUT!
She tells me there are two hot spots on my ribs: One on my anterior of my 2nd rib and one on my anterior 5th rib.
She states she and my doctor spoke with the Radiation Oncologist in charge of the radiation treatment I recently received. (That explains why it took some time to let me know the results.) It was decided that those spots may be from inflammation caused by the radiation to the mass of lymph nodes in the mediastinum--the radiation had to exit the body somewhere. I can continue treatment with Kadcyla! A bone scan will be ordered in three months and will be compared to the CAT scan to be done during the same time period. For now, it is another watch and wait situation.
She also mentioned that she wanted to talk to me before I went to My Chart and read the report. I see why, and so will you. It is not very comforting.
Foci uptake in the anterior left second rib
and anterior left fifth rib
are favored to represent metastases.
I was hoping for another "nothing to see here" moment, but instead, it is nothing more than the old guessing game of cancer.
In the scheme of all things cancer, this is a good report. I am going to believe that this is not cancer spread and do my best not to worry. The ride of doom and gloom that I will eventually have to get on left without me today, thankfully.
In other happy news, a new buddy is coming to live with me. I am so excited!! He will be here on the 11th. Not sure of the name yet. Any suggestions?










